Background
Industry – Medical device
Requirement
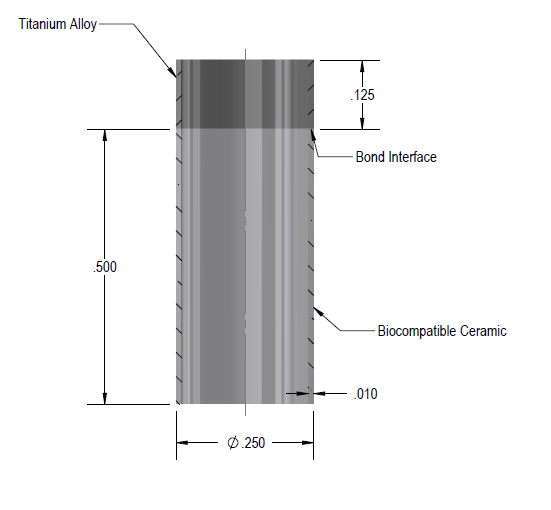
To seal an oxide ceramic and Titanium alloy with nothing in-between. The most common sealing material in medical implant is pure Au. In this situation, however, the FDA approval was based on the direct seal of the ceramic and metal with no Au braze in-between.
Materials Used
Titanium Alloy – FDA Approved (Not specified intentionally)
Ceramic – Biocompatible Stabilized Zirconia
Challenges:
The customer had limited time to get a new implantable medical device to market. Having to approve an additional material at the expense of trial and evaluation costs would have doomed the project such that bonding via brazing was not possible. Faced with time constraints and a non-typical material combination, a disciplined, efficient and innovative approach was required.
Solution:
After a deep level review of the viable options to process these components in the allotted time, IJ Research recommended the best option possible. The decision was to develop a diffusion bonding process in as short as time as permissible which would end up being just a few weeks. Using IJ Research’s material science background to its advantage, the joint was designed to promote the movement of Titanium atoms into interstitial sites of the ceramic lattice structure providing an interdiffusion bonding interface of angstrom thickness. All the required conditions of the component parts for such processing were also completed in the limited timeframe.
Results:
The optimization of the process parameter was successfully completed in the limited timeframe. The customer was able to integrate the design into the product with no need of additional material evaluation, clinical studies, and more importantly no need of any additional FDA approval.
Years later, IJ Research developed a direct bonding process of green body ceramic and metal for hermetic seal applications. Please refer to the Technology section of this website for more information.


No responses yet